April 2023 marked five years since the revolutionary flash glucose monitor, the Freestyle FS Libre, became free for people with type 1 diabetes in Ireland, however, only to those aged between the age of four and twenty-one years, which is approximately 20% of our population.
The FS Libre is a tiny blood glucose monitor worn on your arm which takes a glucose reading every five minutes and sends it to an app on your phone. It reduces the need for a traditional finger-pricking method and provides a lot more information about glucose levels which people with diabetes need to manage their diabetes.
As you can imagine, the type 1 diabetes community reacted to this news with anger and frustration, but more on this later, first I would like to explain what is so life-changing about this device, and then I will give some of the background what happened since January 2018.
Image copyright Abbott, 2018, The Freestyle Libre worn on the arm.
What is so special about this glucose monitor?
The number one reason that sets this monitor apart from the other two available in Ireland is affordability – it costs half of what is the most expensive of the three. However, most people were not aware that devices such as these existed before 2018.
People with diabetes are required to monitor their glucose levels as part of their daily diabetes management to keep them safe. Glucose levels that fall too low can cause unconsciousness as our brains shut down to conserve energy. Glucose levels that are too high over time can cause the complications of diabetes, such as blindness, kidney disease, foot ulcers and amputations.
Since the 1980s, the tool available to do this relied on pricking our fingers to draw blood which was placed on a stick and inserted into a meter that delivered a blood glucose result. While this device recolonised diabetes management forty years ago, there were issues with this method. For example, an accurate result relied on the following:
access to hand washing facilities,
carrying all the accoutrement (the complete test kit involves the meter, the finger pricker device and test strips and not items that I could easily fit in my pockets),
and remembering to check
Since the early 2000s, technology has advanced to replace finger pricks with a wearable sensor that sits under our skin and takes a reading every five minutes, called Continuous Glucose Monitors or CGMs. In addition to this information, the device provides a trend arrow to let me know where my glucose levels are head; up, down or stable! This information is huge for making diabetes care decisions.
The CGMs available before the Libre were extremely expensive and still are. There are a number of these devices in Ireland reimbursed through the HSE’s Long Term Illness Scheme, but up until the Freestyle Libre was launched, most were unaware they existed.
The Background To Libre Access
The FS Libre glucose monitoring system became available to purchase in Ireland in November 2016. There was much fanfare and marketing by Abbott, the makers of the FS Libre. Here's one newspaper article that covered the launch with former Dublin Senior County Footballer Kevin Nolan. It has been available to buy in the United Kingdom since 2014, and if you were lucky enough to have friends and family living there, you could access it through them.
In February 2017, Abbott submitted an application to have the device included on the HSE’s list of reimbursable items, like most of our other medications and glucose test strips, and on the 24th January 2018, five years ago, the HSE announced that only people aged between 4 and 21 years would be allowed access to the reimbursement scheme.
Approximately 28,000 people in Ireland live with type 1 diabetes, and it’s estimated that less than 5,000 of those are between the ages of 4 and 21 years. As I mentioned earlier, the diabetes community reacted angrily to this news and took to our online forums to express this. One woman, Davina Lyon decided to act and created a petition on change.org asking the Health Minister, the Department of Health and the HSE to remove the age restriction. This one act spurred a grassroots advocacy movement. The petition collected over 19,000 signatures within two weeks and was presented to TDs on the 18th of April 2018, after which promises were made to keep this issue active in the Dáil. Promises that were kept.
Presentation of the Freestyle Libre Petition by members of the Diabetes Community and Diabetes Ireland to members of the Dáil in 2018. Photo credit: David Coleman.
Advocacy Activity
Since 2016, there have been hundreds of Parliamentary Questions submitted by TDs (Irish Public Representatives) on behalf of their constituents. All are asking when the FS Libre would be included in the HSE reimbursement scheme for all people with type 1 diabetes. However, no action was taken by the Department of Health or the HSE to address this.
Our national charity, Diabetes Ireland, created two surveys, one in 2019 and another in 2022, which were presented to the HSE as evidence of the effectiveness and the improvement of the quality of life for people with diabetes.
Diabetes Community Calls on Minister for Health to extend Freestyle Libre to all People with Type 1 Diabetes as Survey highlights Huge Quality of Life Improvements in Irish Users (Diabetes Ireland, 2019).
The second survey four years later was created as a part of a submission to the HSE’s Health Technology Assessment HTA to find out the cost benefits of the device.
Survey Highlights Frustration among Adults Living with Diabetes and Diabetes Health Professionals regarding lack of access to Freestyle Libre (Diabetes Ireland, 2019).
Sadly, the company manufacturing the FS Libre, after four years of meeting with the health service, withdrew from the HTA, and the issue was in stalemate.
Activism Momentum Fades as Uptake Grows
A voluntary group of people with diabetes, Thriveabetes, created a webpage on their website dedicated to information about accessing this glucose monitoring. The chatter in the Irish Diabetes Online Community seemed to indicate an increase in uptake of the other CGM devices available. In fact, several people reported that they had been refused an application for the FS Libre, only to be approved for one of the others.
By 2022, there had been hundreds of Parliamentary Questions PQ submitted by TDs (Irish Public Representatives) on behalf of their constituents asking for access to FS Libre, but no one seemed curious about finding out how many people were using these devices. Except for me, that is! I asked a friend to submit a PQ on how many people were being funded through the HSE for these devices and for which ones. I had thought that this information would not be forthcoming, but low and behold, it did.
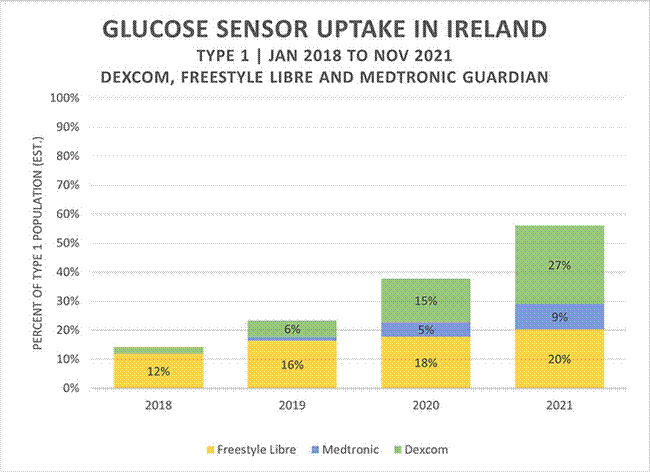
In March 2022, I received responses with how many people were funded by the health service for CGMs and how many were under and over the age of 21 years and wrote a short report based on this information. The report titled "Continuous And Flash Glucose Sensors Uptake In Ireland" was published on my personal blog, Blood Sugar Trampoline. The image above shows how much uptake has increased since 2018.
This information revealed that the HSE funded over 11,000 people with type 1 diabetes for these devices in 2022. That's approximately 42% of the type 1 diabetes population. Over 6,000 adults over the age of 21 years were funded, with most of this group using the more expensive device. Surprise, surprise.
The Minister for Health was asked on several occasions in March and April 2022 in the Dáil if he would remove the age restriction or investigate the current impasse on the progress of this. He responded by requesting the Health Information and Quality Authority (HIQA) to ask if it would consider a system-wide HTA across diabetes care. Sadly, again this HTA did not produce the desired outcome (Oireachtas, 2022).
At this point, the momentum of activism from the diabetes community had slowed down dramatically, especially as there is an alternative to this product available. Unfortunately, the community does still need to campaign for this because sometimes the only choice available may not be the choice that works for you. A lot of people have skin reactions to the adhesive on these products, so they need to be able to try a different product.
Five Years, Two Next-Gen Products Later and Still waiting
Abbott continues to innovate their device and launched Libre 2 in the UK in 2020. The NHS in the United Kingdom made Libre available through the NHS in November 2018 for all people with type 1 diabetes and Libre 3 in 2022, as did most other European countries. Youtuber Kamil Armacky from Nerdabetic demonstrates the improvements in the current version of the Libre in this video.
In April 2022, Abbott, the makers of the FS Libre, submitted an application for the next-generation product, the Libre 2, a process that is supposed to take six months to complete; however, the PQ reply dated January 2023, states that the “Freestyle Libre 2 sensors are currently undergoing formal pricing and reimbursement in line with the Health (Pricing and Supply of Medical Goods) Act 2013. The application remains under consideration with the HSE. The HSE cannot make any comment on possible outcomes from the ongoing process” (HSE.ie, 2023).
April 2023 marked five years since Libre was offered to children and young adults. The diabetes community have been fighting for this for five years now while many other European countries, including the NHS in the United Kingdom, have made access universal.
I am extremely grateful that so many people are accessing these devices in Ireland and that bureaucratic inefficiency and lack of knowledge within the Health Service’s procurement departments aren’t preventing people from doing so.
I’ve been using my CGM since 2015 and could not do diabetes without it. Are you a CGM or FS Libre user? I’d love to hear your story on how you accessed yours; please do share in the comments, or if you are uncomfortable sharing your story publicly, do reach out via email.
Coming soon: an update on the latest figures from the HSE on Flash and CGM uptake in the diabetes population in Ireland.